Aquatic Stressors
My applied research program encompasses the areas of environmental physiology, and toxicology. Our studies are integrative in nature and move from whole organismal to cellular and molecular responses, using a wide variety of techniques. Much of our research is experimental, laboratory based studies but we also perform field based research using native or invasive species. Often our field work is associated with bringing native species into the lab. Our interest is in the study of aquatic species, primarily fish but we have begun to use two aquatic invertebrates, the freshwater hydra and the marine annelid Capitella tellata. In our fish studies, we use a wide variety of species including zebrafish, rainbow trout, arctic charr, lake and round whitefish, and yellow perch. By nature, the research is comparative and integrative.
Impacts of Contaminants in the Aquatic Environment
Much research has documented that human pharmaceuticals are present in our surface waters due to release with wastewater effluent. The release of pharmaceuticals into the environment is a large concern because these compounds are designed with inherent biological activity and physiological pathways are highly conserved across vertebrates. Thus, we expect that pharmaceuticals will have biological activity in aquatic vertebrates such as fish. Our research has focused on four major pharmaceuticals consistently found in wastewater effluent and surface waters: acetaminophen (common analgesic), carbamazepine (anti-epileptic and mood stabilizer), gemfibrozil (lipid regulator), venlafaxine (anti-depressant) and metformin (a type 2 diabetes medication). In the last year, we have begun studies on liquid crystal monomers, electronic waste chemicals that are newly emerging as contaminants of concern.
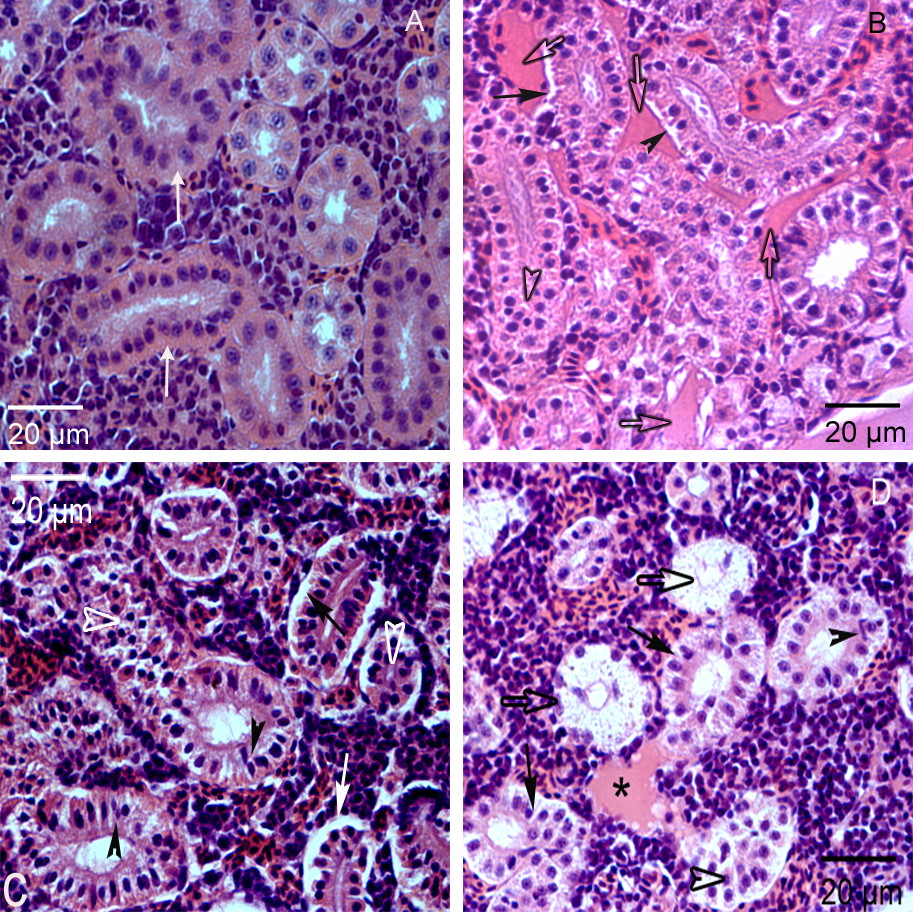
Using environmentally relevant concentrations and chronic exposures, we have exposed zebrafish to single pharmaceuticals, pharmaceutical mixtures and diluted wastewater effluent to assess reproductive, developmental, histological, transcriptomic and multi-generational impacts. We are exploring whether the effects of exposure to these compounds are through their mechanism of action in humans. We use rainbow trout to determine the physiological implications of pharmaceutical exposure in fish. We also determined effects on the brown and green hydra, important freshwater cnidarians! This research uses a wide array of techniques from basic histology and enzyme immunoassays, to microarray and microinjection approaches.
Zebrafish sperm. We are examining sperm morphology and swimming speed as relevant endpoints for male offspring after parental exposure to pharmaceuticals.
Beyond our in vivo studies, we are using the rainbow trout cell line, RTgill-W1, in an acute toxicity assay. This research is focused on examining the potential for replacing the rainbow trout acute lethality test with a cell line test for regulatory use. This is in response to new legislation in Canada, aiming to reduce animal use in toxicology testing.
Thermal Stressor Effects on Fish Development
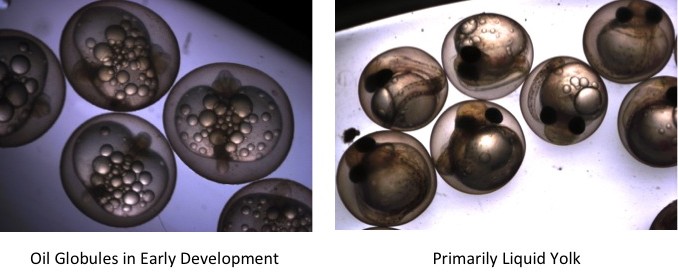
Lake and round whitefish are cold water adapted fish which spawn in late fall. Embryos develop overwinter at very low temperatures, typically between 0 and 2 degrees Celsius. Yellow perch are cool water adapted fish which spawn in spring, developing in warming water. Interestingly, perch do not lay individual eggs but release them in a long ribbon. We capture spawning adults and either generate embryos from in vitro fertilization or allow the fish to spawn in tanks in the lab. The field work is fun but cold! The embryos, like zebrafish, have a clear chorion (equivalent to an egg shell), which allows us to examine them under microscope and see their development in real time.
We study the effects of temperature on developing fish to assess the impacts on embryogenesis and later in life. For these experiments, we manipulate developmental temperature and then move the hatched fish into common garden (i.e. similar) conditions. This research serves two purposes. One obvious purpose is to understand the impacts of climate change. The second, less obvious, purpose is to understand the impacts of once through cooling, a common process used in many industrial processes including power plants. Many effluent discharges are warmer than ambient water and if fish spawn in impacted waters, the embryos can’t move or avoid the elevated temperatures. For direct effects on embryogenesis, we examine changes in development, morphology, survival, heart rate, oxygen consumption, gene expression, and hatching. In the post-hatching phase, we examine survival, time to first foods, behaviour, and thermal preferences. This research will help us to understand the impacts of temperature on the development of important native fishes in the Great Lakes region.
Want to see a great video explaining our whitefish project? Caitlin, an undergraduate from 2016-2017, won best video in the iClimate Video Competition. Check out her video here!
This research has been funded by the Natural Sciences and Engineering Research Council of Canada (NSERC; Discovery, Strategic Project, and Collaborative Research and Development Programs), MITACs, the Canadian Water Network (CWN), Ontario Ministry of Research and Innovation Early Researcher Award program, and industrial partners.